Mechanism Of Action:
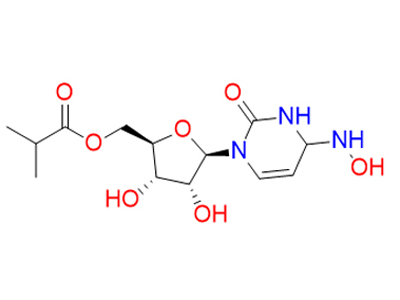
Molnupiravir is hydrolyzed in vivo to N4-hydroxycytidine, which is phosphorylated in tissue to the active 5’-triphosphate form, and incorporated into the genome of new versions, resulting in the accumulation of inactivating mutations, known as viral error catastrophe. A remdesivir resistant mutant mouse hepatitis virus has also been shown to have increased sensitivity to N4-hydroxycytidine.
Indication:
N4-hydroxycytidine and its prodrug are being studied for its activity against a number of viral infections including influenza, MERS-CoV, and SARS-CoV-2.
It is approved in the UK for reducing the risk of hospitalization and death in mild to moderate COVID-19 cases for patients at increased risk of severe disease (eg. with obesity, diabetes mellitus, heart disease, or are over 60 years old).
In the US, it is authorized for emergency use for the treatment of high-risk adults with mild to moderate COVID-19.