oxygen to cells throughout the body. RBC synthesis takes place in the bone marrow, under the control of erythropoietin (EPO) produced by interstitial fibroblasts in the kidney. Anemia can lead to shortness of breath, cognitive dysfunction, significant fatigue, and decreased quality of life, which is associated with increased risk of cardiovascular complications, hospitalization, and death. Severe anemia is common in patients who are dealing with cancer, CKD, inflammatory diseases, myelodysplastic syndrome (MDS) and other serious health conditions. Anemia is a life threatening public health condition which specially affects young children and pregnant women. According to World Health Organization (WHO) estimates, 1.62 million people are affected by anemia, which is about 24.8% of the population globally. The highest prevalence tends to be in children less than 5 years of age, women of reproductive age and pregnant women. According to a global estimate, around 42percent of preschool-age children (i.e. those aged 6–59 months) and 40percent of pregnant women worldwide are dealing with anemia.Roxadustat could be the first in a new class of treatments called oral HIF-PH inhibitors that promotes RBC production or erythropoiesis, through increased endogenous production of EPO. Roxadustat is also in clinical development for chemotherapy-induced anemia and anemia related to MDS. Roxadustat is approved in China, Japan, Chile and South Korea, for the treatment of anemia in CKD patients. In Europe, It’s under final regulatory review following a positive EU CHMP opinion in June 2021.
Roxadustat is being commercialized and developed for the potential treatment of anemia within the US and other countries within the Americas and Australia, New Zealand and China, as well as Southeast Asia, and also is being worked for the potential treatment of anemia in Europe, Russia, Japan, Turkey and also the Commonwealth of Independent States, South Africa and therefore the Middle East.
Hence, one of Parsian’s fields of activity is research and development in Anemia to manufacture active pharmaceutical ingredient (API) of Roxadustat with the highest global standards to help patients who are battling with Anemia to have fewer concerns by the latest guidelines and with the lowest adverse effects.
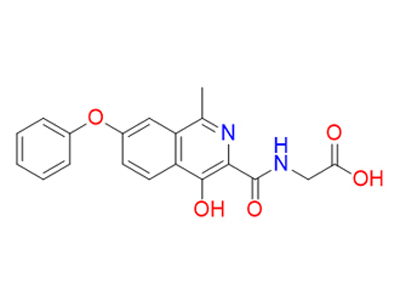
Anti-Anemic
Cas No: 808118-40-3
Mechanism Of Action: Anemia is a common complication of chronic kidney disease that can be caused by reduced production of renal erythropoietin (EPO), functional iron